Disease management of intractable conditions, like Refractory Epilepsy and Dementia, as well as the recent spike in COVID 19 around the US, is driving healthcare providers and researchers to seek alternatives to existing drugs or protocols.
MGC’s track record developing botanical or bio-pharmaceutical solutions as alternatives to existing medications, many of which have negative side effects affecting patients’ quality of life, made this an exciting collaboration for AMC. We look forward to introducing MGC’s products to our medical community.
26 August 2021
ASX Code: MXC
LSE Code: MXC
MGC Pharmaceuticals Ltd.
MGC Pharma executes binding US$24 million supply and distribution agreement for product launch into the USA
Key Highlights:
- MGC Pharma has executed a binding, 3-year U.S. market Supply and Distribution Agreement with U.S. based Company, AMC Holdings Inc (AMC), with minimum orders of US$24 million of MGC phytomedicine products including CannEpil®, CogniCann® and CimetrA™.
- This is MGC Pharma’s first USA supply agreement dedicated to taking MGC’s pharmaceutical phytocannabinoid products into the world’s largest healthcare market.
- US$3 million order for CannEpil®, CogniCann® and CimetrA™ for Year 1 to be placed 5 days from grant of a National Clinical Trial Number, with an additional US$21m of orders over years 2 and 3.
- For the Year 1 minimum order, AMC will pay MGC Pharma US$750,000 in advance prior to the receipt of the products, followed by an irrevocable US$2.25 million Letter of Credit.
- AMC has been founded by leading U.S. Healthcare and ex-Federal Government legislative and regulatory executives, as a specialist vehicle for the import and distribution of specialist phytomedicines into key U.S. markets.
- AMC is seeking to expand the research and growth of phytomedicines in the USA, and view MGC Pharma as the leader in the sector.
- Clinical trials in the U.S. for CannEpil®, CogniCann® and CimetrA® will be initiated by AMC and leading research institutes in the U.S., subject to Global Ethics Committee approval, as additional sites to the ongoing trials in Israel and Australia.
- AMC will seek U.S. regulatory approvals for all products which they intend to distribute, including import licenses, as well as undertaking clinical trials in the U.S., all the way through to U.S. Food and Drug Administration (FDA) approval.
- AMC and MGC will first seek approval to distribute and issue CannEpil® to patients via Florida’s Early Access Scheme.
MGC Pharmaceuticals Ltd (‘MGC Pharma’ or ‘the Company’), an EU based bio-pharma company specialising in the production and development of phytocannabinoid-derived medicines, is pleased to announce that it has signed a binding U.S. Market Supply and Distribution Agreement (U.S. Supply Agreement) with U.S company, AMC Holdings Inc (AMC), with minimum orders of US$24 million for MGC products including CannEpil®, CogniCann® and CimetrA™ over the initial 3 year period.
This is the first dedicated supply agreement executed by MGC Pharma for the supply of MGC Pharma pharmaceutical products into the USA, the largest healthcare market in the world. The Agreement with AMC is also an important step in expediting the clinical trials process for both CannEpil® and CogniCann®, as well as providing access to these medicines to more patients.
As the licensed distributor of MGC Pharma products in the USA, AMC will undertake all marketing activities in the U.S., as well as managing the import and warehousing of the products. To assist with product development activities, AMC will also collect data from the end users of the products for analysis by MGC Pharma.
The U.S. Supply Agreement includes a minimum US$3 million of sales in Year 1, subject to AMC receiving a National Clinical Trial Number (NCTN) for a MGC Pharma product by the end of September 2021. Obtaining the NCTN will enable hospitals in the USA to participate in the ongoing clinical trials for CannEpil® or CogniCann® under approval of the Global Ethics Committee. With AMC are already well advanced in this registration process.
U.S. based clinical sites will be added to the global clinical trial program for CimetrA® in order to initiate the registration process for CimetrA® in the U.S. AMC and MGC will first seek approval to distribute and issue CannEpil® to patients via Florida’s Early Access Scheme.
AMC Holdings, Inc.
AMC Holdings, Inc. is a privately held, U.S. based distribution and marketing company, recently founded to bring cutting edge bio-pharmaceutical products currently in clinical trials or commercial production overseas, into the U.S. healthcare marketplace.
AMC has been founded to arrange for leading U.S. researchers, academic institutions, and physicians to join existing clinical trials abroad, and establish U.S. based trials for promising bio-pharmaceutical products that meet three key criteria:
- Address disease management for pressing healthcare needs that have proven intractable and expensive with existing treatments or protocols
- Offer botanical or bio-pharmaceutical solutions where none exists, or as alternatives to existing medications with negative side effects affecting patients’ quality of life; and
- Offer the prospect of expanding access to novel treatments with lower cost alternatives to existing drugs or protocols
- CannEpil®, CogniCann® and CimetrA™ all meet AMC’s key criteria, which have paved the way for the Supply and Distribution Agreement with MGC Pharma.
AMC was founded and is managed by a Board and Executive with extensive links to U.S. Federal governmental institutions and organisations, and hospitals with decades of experience in the regulatory and political capitals throughout North America, which will be vital in enabling MGC Pharma’s products to be distributed and marketed across the USA.
Their Management Team and Advisory Board include:
· CEO Brett Scott, a former legal trial attorney in the Tax Division and a Senior trial attorney in the U.S. Justice Department’s Civil Division. He later joined the staff of former U.S. Senator Conrad Burns (R-MT) as general counsel. Brett was later recruited by U.S. Senator John McCain (R-AZ) to serve as legal counsel to the Senate Subcommittee on Communications.
· Former U.S. Attorney Bobby O’Neill who once headed the Justice Department’s Narcotic and Dangerous Drug Section.
· A Drug Enforcement Agency Lifetime Achievement Award winner, Tampa attorney James Cusack.
· General Counsel Brent Yessin, who has represented major medical centres, academic teaching hospitals and health systems around the world,
(Additional information about AMC Holdings and its board can be found at the end of this Announcement).
U.S. Supply Agreement Key Terms
The key commercial terms of the U.S. Supply Agreement between MGC Pharma and AMC are detailed below:
· Binding agreement executed for an initial term of 3 years from the establishment of a U.S. trial site, with an option for AMC to extend for a further 3 years on satisfaction of milestones including regulatory approvals and contracted order quantities.
· Exclusive U.S. import and distribution rights for a range of MGC Pharma products including CannEpil®, CogniCann® and CimetrA™.
· Contracted minimum orders of US$24m over the initial 3-year term of the Agreement, with orders to be paid for in advance of supply, except for the initial US$3m order, of which US$750,000 will be paid in advance, with the remaining balance to be covered by an irrevocable Letter of Credit acceptable to MGC. The Letter of Credit can be drawn down by MGC on the anniversary of the Agreement.
· AMC to facilitate the application for regulatory approvals and certifications to sell MGC Products in the U.S. and its territories, including the establishment of clinical trials.
· MGC has the option to take a board position on AMC
· The Agreement may be terminated at the election of MGC upon failure of AMC to secure regulatory approval for the import and prescription for at least 1 of the listed MGC products by 30 September 2021, evidenced by the issue of a National Clinical Trial Number, or failure to meet agreed minimum annual orders, of:
- Year 1: US$3,000,000
- Year 2: US$6,000,000
- Year 3: US$15,000,000
- 100,000 units per month, from FDA approval of a product.
· AMC to be paid a marketing fee to facilitate product promotion and education in the U.S. of 10% of product list price.
· MGC Pharma will have the right to receive a 10% equity interest in each of AMC’s regional operating companies, with anti-diluted protection.
· MGC Pharma to grant AMC options over new shares in the Company on the following terms:
o Grant of options on satisfaction of the following conditions:
– No stated termination event; and
– MGC Pharma granted 10% equitable interest in AMC regional operating company’s
o Unlisted options to be granted, based of the Company’s share price at date of issue following completion of conditions-
– €1,000,000 of MGC options with an exercise price of £0.05
– €1,000,000 of MGC options with an exercise price of £0.08
– €1,000,000 of MGC options with an exercise price of £0.12
– €1,000,000 of MGC options with an exercise price of £0.15
– €1,000,000 of MGC options with an exercise price of £0.18
o Vesting conditions:
– The option tranches will vest upon AMC meeting Year 1 and 2 total sales targets of US$9m
o Options expire 4 years after grant date
The Products
Initially, the agreement between MGC Pharma and AMC will focus on the following products: CannEpil®, CogniCann® and CimetrA™
CannEpil® is a phytocannabinoid derived IMP, designed to treat Drug Resistant Epilepsy with a high CBD, low THC formula. The Phase IIb clinical trial for CannEpil® has regulatory approval at the Schneider Children’s Medical Hospital in Israel and will focus on the safety and efficacy of CannEpil® as an add-on treatment for children and adolescents with treatment resistant epilepsy, also known as Refractory Epilepsy. This randomised placebo-controlled Phase IIb trial is targeting the recruitment of more than 100 patients.
The agreement with AMC follows on from CannEpil® being added to the Primary Care Reimbursement Service in the Republic of Ireland (see announcement 14 June 2021), making it free of charge for Irish patients prescribed the treatment under the Medical Cannabis Access Program.
Approximately 50 million people worldwide suffer from epilepsy, with approximately 33% of adults, and 20-25% of children, suffering from Refractory Epilepsy (or Drug Resistant Epilepsy), which cannot be controlled with traditional anti-seizure medication, highlighting the size of the market for CannEpil®.
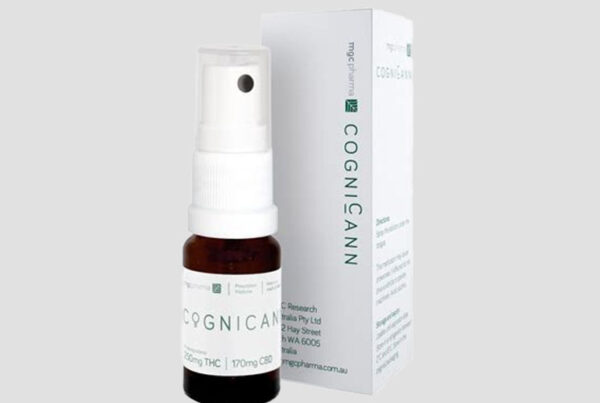
CogniCann® is a formulation of phytocannabinoids that has been developed with the specific aim of treating the symptoms of Dementia and Alzheimer’s. CogniCann® is currently undergoing a Phase II clinical trial at the University of Notre Dame in Perth, Western Australia which has 21 patients currently enrolled, and is designed to evaluate the potential behavioural benefits of CogniCann® on patients with Dementia and Alzheimer’s disease.
CimetrA™ is a nano-micellar pharmaceutical synergetic composition consisting of Curcumin, Boswellia, Artemisinin, and optionally Cannabinoids and/or Nitroxides. The composition can be manufactured in liquids or in solid pharmaceutically acceptable carriers, has antioxidant, anti-inflammatory, immuno-modulating and anti-viral properties and can be designed for multiple therapeutic applications utilising self-nanoemulsifying drug delivery systems.
Preclinical and clinical results to date support the pharmaceutical composition of CimetrA® as an effective treatment for addressing anti-inflammation and Cytokine over-production (known as Cytokine Storm) in all tested COVID-19 patients.
Roby Zomer, Co-founder and Managing Director of MGC Pharma, commented: “This is an important milestone agreement for MGC Pharma, as it provides MGC access to the largest healthcare market in the world. We look forward to working with our new partners at AMC and utilising their expertise and network to widen patient access to MGC’s phytomedicine products.
This Agreement provides MGC Pharma with a pipeline for strong revenue streams over the next three years, with the possibility of larger revenues to follow, and the opportunity to be at the forefront of phytomedicines in the USA. Additionally, the ability to add new sites to our current clinical trials programmes for CannEpil® and CogniCann® will greatly enhance the ongoing process for both. I personally would like to thank all at MGC Pharma and AMC who have made this possible and look forward to sharing our progress with investors in due course.”
Brent Yessin, AMC’s General Counsel and President of AMC Florida, agreed, “We believe that disease management of intractable conditions, like Refractory Epilepsy and Dementia, as well as the recent spike in COVID 19 around the U.S., is driving healthcare providers and researchers to seek alternatives to existing drugs or protocols. MGC’s track record developing botanical or bio-pharmaceutical solutions as alternatives to existing medications, many of which have negative side effects affecting patients’ quality of life, made this an exciting collaboration for AMC. We look forward to introducing MGC’s products to our national medical community.”